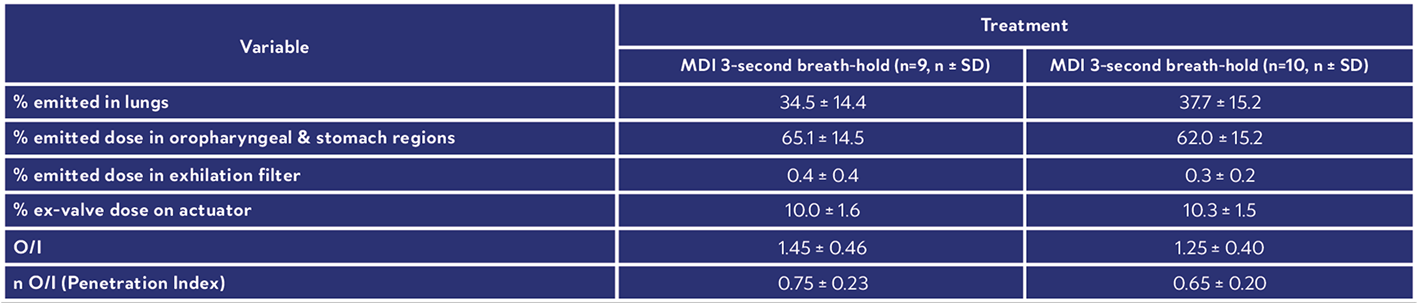
This scintigraphy study was the first to evaluate the lung deposition of a novel fixed-dose triple combination pMDI using co-suspension technology (Israel et al., 2020). During the study, each healthy volunteer underwent a 81mKr gas ventilation scan to define the lung margins (Figure 3a) and a 57Co transmission scan to determine regional tissue attenuation (Figure 3b). Simultaneous anterior and posterior views of the lungs and stomach, and head/neck were performed immediately post dose, and regional lung deposition i.e. inner (I) and outer (O) regions of interest (ROI), were assessed. Figure 3a shows the I & O regions superimposed over the ventilation image. This novel fixed dose triple combination (ICS, LAMA, LABA) pMDI consistently delivered a high lung dose (see Table 1). Results showed similar regional depositions for each breath hold manoeuvre (Figure 4), suggesting the length of breath-hold had no significant effect on total lung and peripheral deposition patterns of this novel co-suspension delivery system (Table 1). It was concluded that this product is suitable for use by asthma and COPD patients who may not be able to perform long breath holds.Case Studies
Radiolabelling Objective
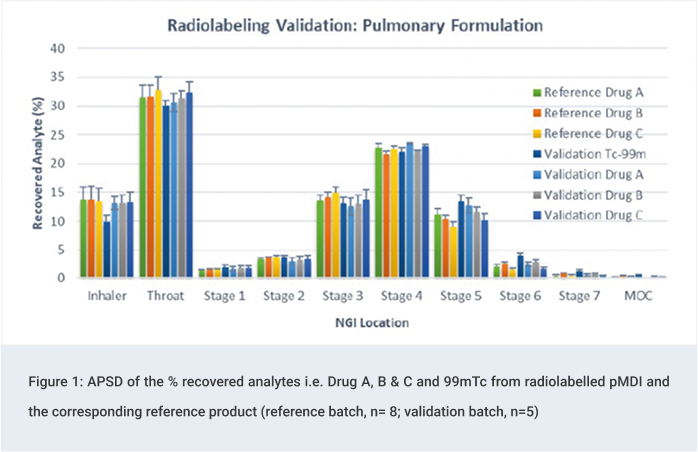
Radiolabelling Validation
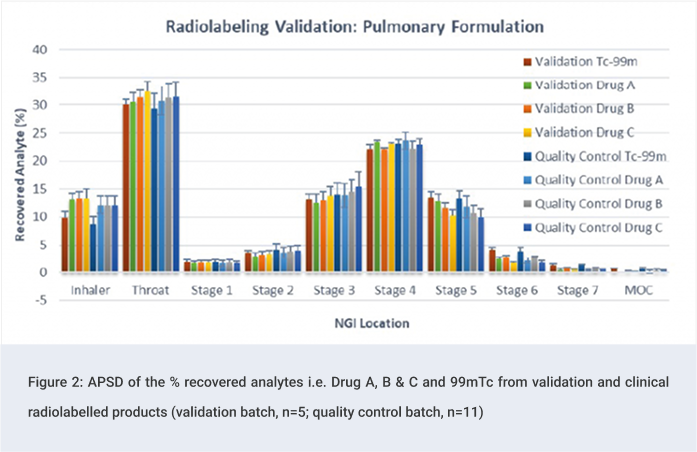
Quality Control Tests: Clinical Phase
Clinical Study
On the dosing day, subjects were administered a single dose i.e. two inhalations from the radiolabelled pMDI, followed by a 10 or 3 second breath hold depending on randomisation.
The study was conducted in compliance with the standardisation guidance published by ISAM (Newman et al., 2012).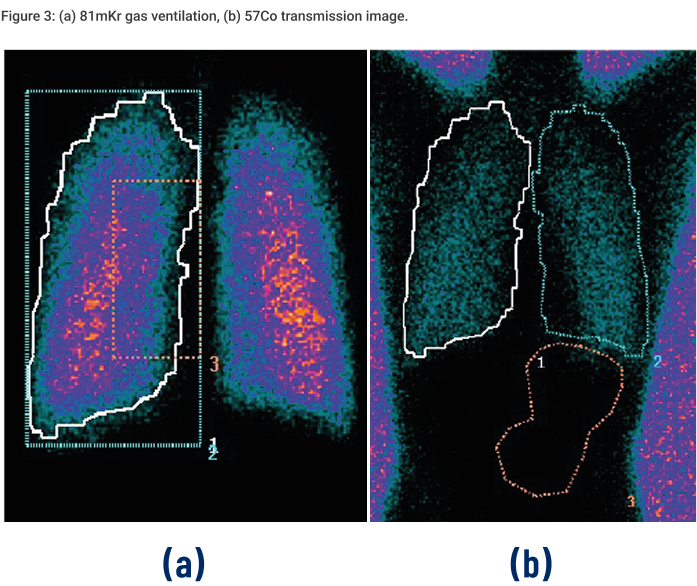
Results
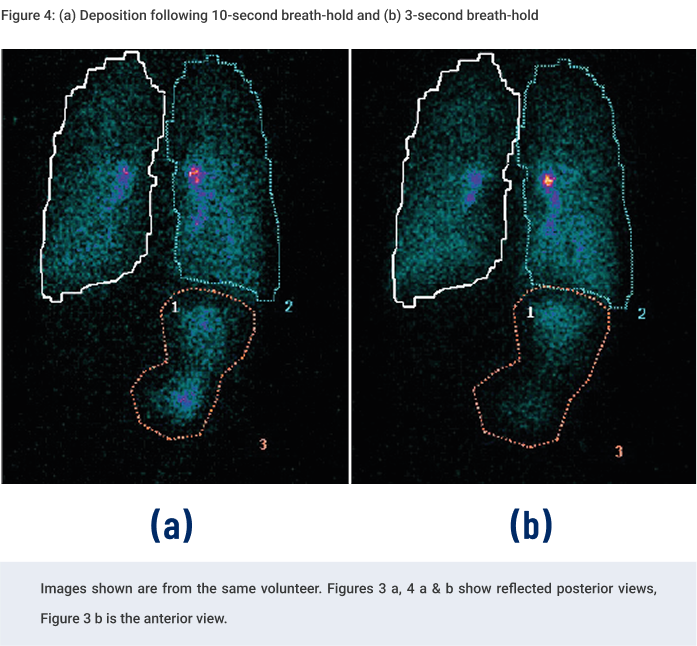
Table 1: Summary Of Derived Deposition Data
Conclusions
References
